3D bioprinting is an additive manufacturing process where organic and biological materials such as living cells and nutrients are combined to create artificial structures that imitate natural human tissues.
In other words, bioprinting is a type of 3D printing that can potentially produce anything from bone tissue and blood vessels to living tissues for various medical applications, including tissue engineering and drug testing and development.
Perhaps the most significant driver of 3D bioprinting is regenerative medicine. According to Chris Mason and Peter Dunnill, this involves replacing or regenerating “human cells, tissue, or organs, to restore or establish normal function.” Here, bioprinting can have a central role, especially considering the high demand for organ and tissue transplants worldwide.
In this article, we’ll explore the fascinating and futuristic technology known as 3D bioprinting. We’ll cover its most important aspects, including its main applications, how it works, and how it’s envisioned to work in the future.
Before jumping into the article, it’s worth noting that very few 3D bioprinting applications are ready for patient use, and though the future looks very promising, you shouldn’t get your hopes high on having a new liver or heart produced for you just yet.
Main Applications
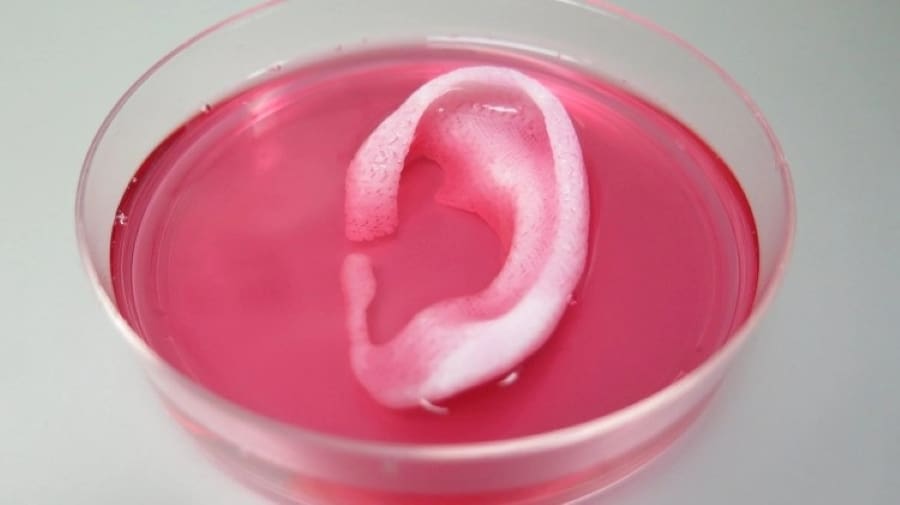
The products obtained from bioprinting technologies can mimic both the biological and functional properties of our bodies’ natural-occurring structures and tissues. This can potentially lead to different kinds of applications, but today there’s only one feasible use for bioprinting: pharmaceutical drug testing and research.
While the ultimate goal of 3D bioprinting is the production of artificial organs for transplantation (as we’ll see next), the complexity involved in making them function as real organs is huge. However, scientists today can successfully create biological structures and tissues that imitate natural ones.
So, instead of bioprinting fully functional kidneys, researchers can already create structures that chemically behave like kidney tissue. While far from the original goal, these structures can be used to test new drugs without having to rely on real-life patients that could suffer from unexpected side effects.
Besides the ethical part of it, drug development with bioprinted materials can make pre-clinical trials of new drugs much more cost-effective, helping them to be validated and reach the market sooner, while also potentially reducing the need for animal testing.
Future Applications
Yet, 3D bioprinting started as a regenerative medicine tool. The production of artificial organs for transplantation would solve the issues of high demand and low availability, as well as post-surgical complications associated with organ rejection given that the fabricated organs would be developed using the patient’s own organic material.
While organ replacement is the ultimate objective, in the meantime, tissue repair has been showing very promising results. Instead of creating entire functional organs, the small tissue patches can be potentially used to regenerate and treat organs like the liver and heart. Bone and skin grafting can also benefit from the technology, including surgery for reconstructive and aesthetic purposes.
Both these applications associated with regenerative medicine are still in development, with only a handful of successful cases in research labs and animals such as the University of Toronto’s project of “printing” skin on burn injuries.
How Does It Work?

In essence, 3D bioprinting works similarly to conventional 3D printing: digital models are transformed into physical three-dimensional objects via layer-by-layer fabrication techniques.
Several bioprinting methods are available, including those based on extrusion, where materials are discharged from a needle to create layers, and laser-assisted technologies, where the laser acts as a heat source to help deposit materials onto the substrate, in a process similar to selective laser melting (SLM).
Regardless of the bioprinting method, instead of inorganic raw materials such as filament, resin, and metal, here, the feedstock material is composed of biological agents and living cells.
These materials are known as bioinks, which are mainly composed of living matter like cells within a carrier material – like collagen, gelatin, hyaluronan, silk, alginate, or nanocellulose – that act as a molecular scaffold for the structure to grow and nutrients to provide support.
In general terms, the bioprinting process as a whole involves several steps which can be summarized in three key stages: the preparation, which consists of creating the digital model (analogous to the 3D modeling stages of conventional 3D printing); the layer-by-layer construction process itself; and the post-bioprinting stages that involve mechanical and chemical stimulation to stabilize structures and mature biological material.
In the next section, we’ll detail the individual steps of a general 3D bioprinting workflow.
Key Steps of Bioprinting
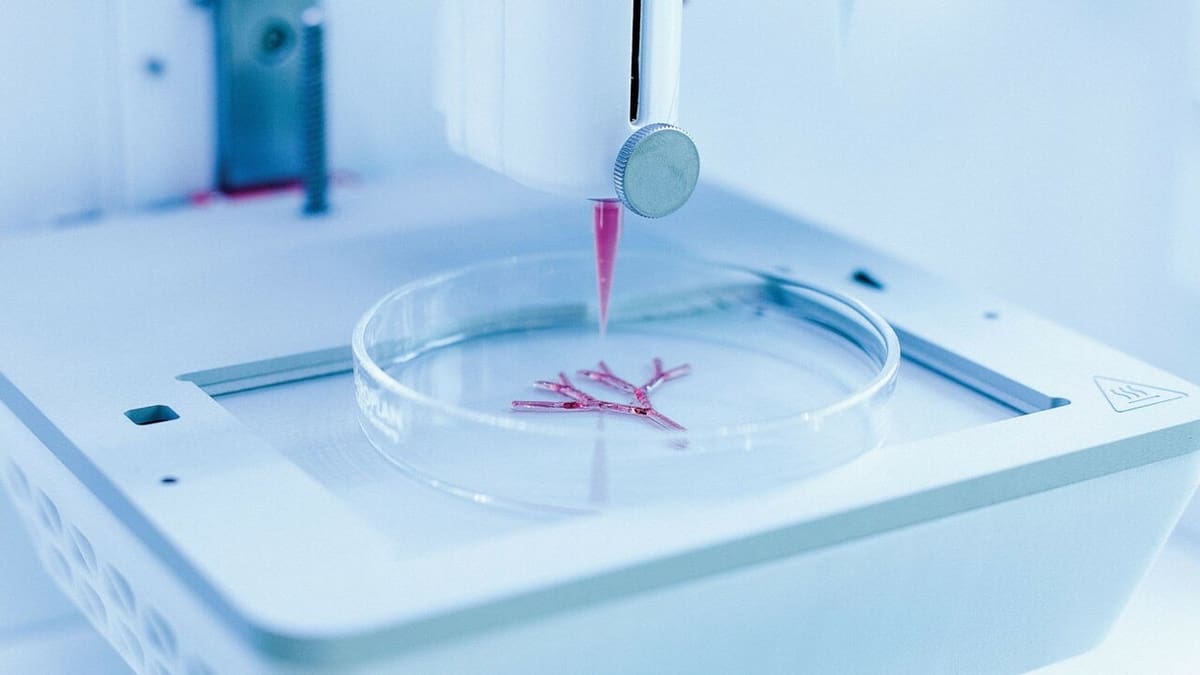
Despite the various 3D bioprinting types and methods, a typical process follows roughly the same standard series of steps.
Preparation
- 3D imaging: Similarly to what 3D scanning is to conventional 3D printing, normal computed tomography (CT) and magnetic resonance imaging (MRI) scans are utilized to get as much information as possible about biological structures and their surroundings.
- 3D modeling: The actual three-dimensional model of the structure or tissue is generated by special software. The model is developed on a microscale, already considering the layer-by-layer fabrication method and the carrier materials.
- Bioink preparation: The bioink type and preparation depend on the printing method of the particular bioprinter. For instance, in extrusion-based bioprinting methods, the bioink should be a highly viscous fluid to keep a relative structure after extrusion, whereas for other methods such as inkjet or those involving microfluidity, the feedstock materials must be in a more liquid state in to flow properly.
Construction (or 3D printing)
- Printing: Regardless of the method, the 3D bioprinting process involves depositing the material layer-by-layer. The printing resolution can reach incredible single-cell deposition in some bioprinting methods, like Fluicell’s microfluidic flow printing.
Post-Bioprinting
- Crosslinking: Once printed, the materials are still in a relatively sluggish state, so an extra step is necessary to properly solidify and blend them together. This is known as crosslinking, and it’s an essential step to ensure the mechanical and chemical properties of the printed structure. Crosslinking can use different environmental controls like UV light, temperature, and chemicals, among others.
- Maturation: Finally, the bioprinted and crosslinked structures need to grow – biologically. This means that the printed living cells will reproduce, and tissue will grow following the underlying printed structures. This step is also called incubation and is done inside bioreactors that create a favorable environment for reproduction and tissue growth.
3D Bioprinters
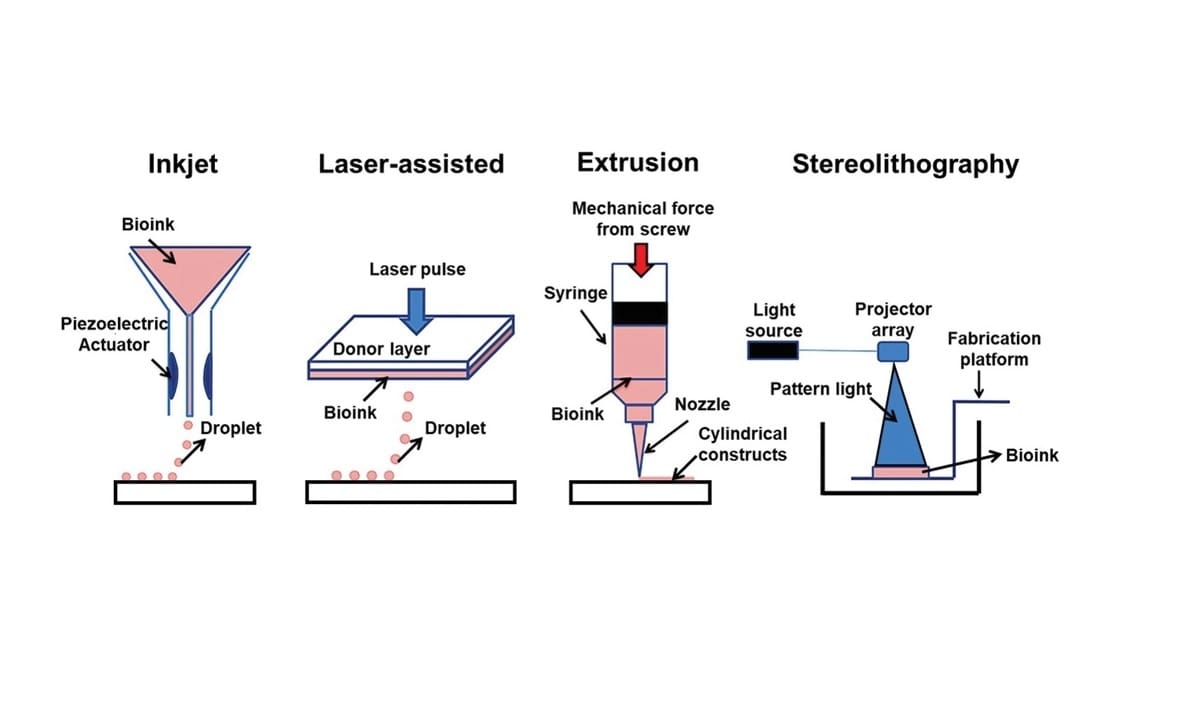
It may come as a surprise to many that the market for 3D bioprinters isn’t small. For starters, there are different bioprinting methods, which can be divided into four main categories: extrusion-based, inkjet-based, laser-assisted, and stereolithography.
Each method offers different pros and cons, and each is better suited to some bioprinting purposes over others. From modest desktop machines to massive bioprinting stations, there’s a great variety in equipment size, functionality, and purpose. Let’s go over a few bioprinters to better illustrate the idea.
Take, for example, the Allevi bioprinter series developed by the American company that goes by the same name. These are small extrusion-based desktop printers that use small syringes to deposit bioinks and hydrogels with incredible precision (up to 1 μm).
Moving up the ladder in terms of complexity, the NGB-R bioprinter by French company Poietis combines different bioprinting deposition methods in one single technology that includes laser-assisted and extrusion-based techniques. This bioprinter is focused primarily on producing artificial human tissues for research and development.
Last but definitely not least, the bulky Dr. Invivo 4D6 by Korea-based Rokit is a full biofabrication station that includes a UV chamber sterilization, an optical microscope, a built-in cell incubator for maturation, and an optional frozen build plate that can reach -30 °C. And yes, it can also do extrusion-based 3D bioprinting.
As you can see, the bioprinter market is very diverse.
Recent Developments

While we’re still quite far from mass-producing artificial organs and human tissues, it’s not unusual to hear about new, exciting developments in the biofabrication area over the past years.
In April 2019, news media outlets worldwide reported the first vascularized 3D printed miniature heart developed by Tel Aviv University‘s School of Molecular Cell Biology and Biotechnology. The mini-organ was created using patient-specific materials but showed no functionality whatsoever.
However, just a few months after the Israeli researcher’s breakthrough, the American biotech company BioLife4D announced its own bioprinted heart that was bigger and replicated some functionality found in human hearts.
Another massive development in organ bioprinting was achieved by the researchers at the Rensselaer Polytechnic Institute in New York. In 2019, they were able to create fully-vascularized skin patches. The skin is the human body’s largest organ, and the artificial patches made from the patient’s own cells would enable fast treatment for severe burn victims. The team is now focused on developing the transplant and integration of the patches’ vascular system directly into the patient’s body.
What Does the Future Hold?

With a growing market of bioprinters and an increased interest in tissue engineering and regenerative medicine, it’s clear that bioprinting technologies will continue to develop even more rapidly.
It will indeed justify its value both from a moral and ethical perspective, which is always a significant challenge in technologies associated with medicine and nature. Let’s see how the technology will develop in a few more decades.
In the meantime, be sure to check other articles about 3D printing and healthcare, including prosthetics fabrication and medical devices.
License: The text of "What Is 3D Bioprinting? – Simply Explained" by All3DP is licensed under a Creative Commons Attribution 4.0 International License.



