The technology that lets us see inside the human body (CT scans, MRIs, ultrasounds) has grown increasingly sophisticated over the years. But our ability to create accurate 3D models from that data — used for surgical planning, education, and tools — is still a time-consuming and complex process.
Converting patient scan data into a 3D digital or printable file takes a substantial amount of tech know-how and medical expertise. One company, Belfast, Irleand-based Axial3D, offers software that, with AI and algorithms, automates and accelerates the process.
“Our mission is to make patient-specific 3D solutions accessible to all, enabling surgeons, radiologists, and engineers the resources to improve patient outcomes and accelerate patient-specific programs,” the company says.

This week, the US Federal Drug Administration approved Axial3D’s Insight Medical Image Segmentation Platform for use in orthopedic, maxillofacial, and cardiovascular applications.
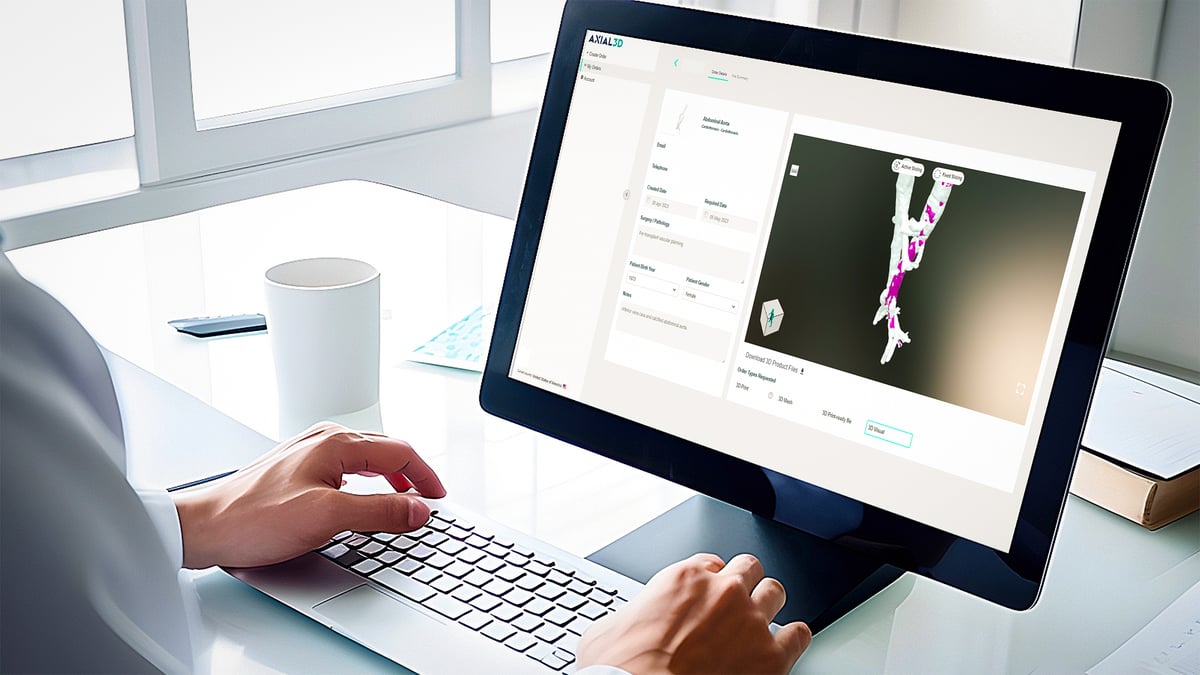
Insight automates the conversion process of 2D medical images into accurate 3D visualizations and 3D print-ready files. The models can also be used for patient education, practice surgeries, as well as to size or pre-fit medical equipment.
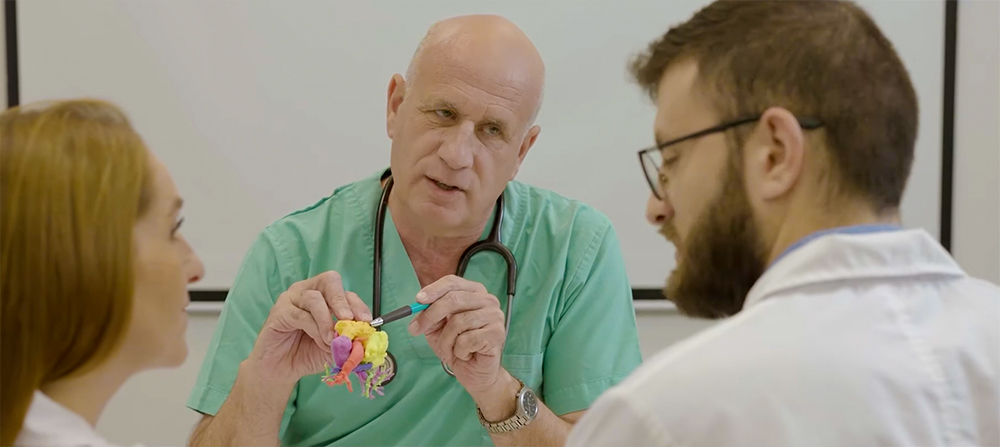
Patient-specific medical models have been shown to cut hospital costs, better prepare doctors for procedures, and improve outcomes for patients. A study in 2020 found an average operating room time savings of 62 minutes per case and a cost savings of $3,720 per case when 3D printed anatomical models were used to prepare for orthopedic and maxillofacial surgery.
The FDA considers 3D printed anatomical models that can affect or change diagnosis, patient management, or patient treatment as diagnostic tools and, as such, are Class 2 medical devices. Models for diagnostic use must be prepared using software that also has received FDA clearance for specific applications.
Insight’s FDA clearance is expected to provide a major boost toward scaling-up production processes, the company says, particularly by medical device companies. This 3D data is used to design personalized medical devices beyond models for surgical planning. It aids in developing unique surgical guides used during procedures.
Insight is already used by surgeons, clinicians, radiologists, and engineers to securely upload DICOM images to the platform, where they are automatically segmented and validated to generate a patient-specific 3D file.
This is the second FDA clearance Axial3D has received for Insight, and a “significant milestone for the healthcare industry in its embrace of automation and artificial intelligence to personalize patient care,” the company says.
By automating the traditionally arduous task of manual or semi-manual image segmentation, Axial3D enables healthcare providers and medical device companies to save hours or days per case. Insight’s recent FDA approval is also for orthopedic trauma uses, where time is critical.
The models and guides generated by Axial3D software can be 3D printed on a variety of printers, including the Stratasys Digital Anatomy 3D printers. Stratasys became a major investor in Axial3D late last year. Axial3D also offers a print-on-demand service for models.
Insight is not alone in the market. Software solutions, such as Materialise Mimics InPrint, Oqton’s D2P (DICOM-to-PRINT), and Ricoh 3D for Healthcare, also convert patient data into 3D files for a range of FDA-approved uses.
License: The text of "3D Printing Patient-Specific Medical Models Just Got Easier" by All3DP Pro is licensed under a Creative Commons Attribution 4.0 International License.
