No field showcases the possibilities of 3D printing like medical technology. Since 2018, Evonik has been driving medical 3D printing by offering the industry’s most extensive portfolio of 3D-printable biomaterials for medical technology, which can be used to manufacture medical device parts designed for temporary and permanent body contact.
Among the many developments in today’s medical technology, three very exciting areas are offering a wide range of growth and potential in the PEEK (polyether ether ketone) biomaterials field: the need for more patient-specific and point of care treatment, digitalization and robotics, and metal-free solutions. Evonik supports these areas with a focus on new PEEK solutions to enable 3D printing of individual medical devices.
“Improved, patient-specific treatment at point of care is true reality. For example, it is already possible today to integrate bioactive additives with biomaterials, giving 3D printed implants a range of characteristics that promote faster bone-healing and thus better patient recovery,” said Marc Knebel, head of Evonik’s Medical Systems market segment. “Besides additive manufacturing of individual implants, patient-specific treatment also means to offer material solutions for people’s different natures. In this regard, metal-free solutions like polymer-based medical devices are in demand.”
Digitalized Technology and Smart Biomaterials for Patient-Specific Treatments
A pioneer in polymers for additive manufacturing, commonly known as 3D printing, Evonik was the first company in the world to develop an implant-grade, polymer filament for 3D printable medical implants. Having since then continued to develop in this area, Evonik now offers the most comprehensive portfolio of medical grade PEEK filaments for use in medical-device applications. The filaments are suitable for short- to long-term contact with the body.

The use of easy-to-handle implant grade, 3D printable PEEK filaments – such as VESTAKEEP® i4 3DF – in combination with suitable dedicated medical printers and the integration hardware and software in digital workflows, has led to a positively disruptive step towards point of care treatment: Such a combination of material and technology allows implants to be created directly in the hospital. Having been first achieved with VESTAKEEP® i4 3DF at Skåne University Hospital, a Swedish hospital, in 2021, the potential benefits of 3D printing smart biomaterials on-site has become limitless.
Another breakthrough in patient-specific care with smart biomaterials is the use of the VESTAKEEP® i4 3DF filament early in 2023, in the first US surgeries of a 3D printed spinal implant. Printed by US-based technology company, Curiteva, the high-tech implant was cleared by the US Food and Drug Administration and was the world’s first 3D-printed, fully interconnected porous PEEK-implanted structure of its kind for commercial use.
Recently, the first patient at the University Hospital Basel received a 3D-printed implant based on Evonik’s PEEK filament from in-house production. A 46-year-old man suffered a stroke in 2019. During treatment, his skullcap was removed and replaced. But after a few months, the ceiling began to disintegrate and the skull sank in. A team led by Raphael Guzman, head of neurosurgery, and Florian Thieringer, head of oral and maxillofacial surgery, succeeded in producing an artificial skullcap in the in-house 3D printer.
“Such 3D-printable implant materials are truly breakthroughs. In addition to serving as spinal implants, they open up exciting new possibilities in individually adaptable, medical treatments. Smart biomaterials are engineered for the utmost biocompatibility, biostability and x-ray transparency. By design, these materials are ideal for applications like orthopedic and maxillofacial surgery,” said Marc Knebel, head of Evonik’s Medical Devices & Systems market segment.
Advanced Osteoconductive Medical Plastics for Improve Patient Care
For certain medical procedures, the ability for bone cells to adhere to implants, also known as osteoconductivity, is critical for good patient recovery. Specific ceramics and porous metals are traditional materials used for this purpose. Key to success is a material’s ability to be formed to mimic bone structure and chemistry. Evonik’s VESTAKEEP® Fusion materials offer the ideal “smart biomaterial” solution for this highly complex field. These polymers come in granulates, semifinished stock-shapes and filaments. Smart biomaterials combine multiple features in one material. Of the many defining features of highly advanced VESTAKEEP® Fusion smart biomaterials, the most prominent is its inclusion of a special additive, biphasic calcium phosphate (BCP). BCP has the ability to positively influence osteointegration with the body’s existing natural bone. As a result, bone fusion is promoted, leading to accelerated patient recovery. Characteristic of PEEK-based products, VESTAKEEP® Fusion smart biomaterials can be milled, injection molded and 3D printed.
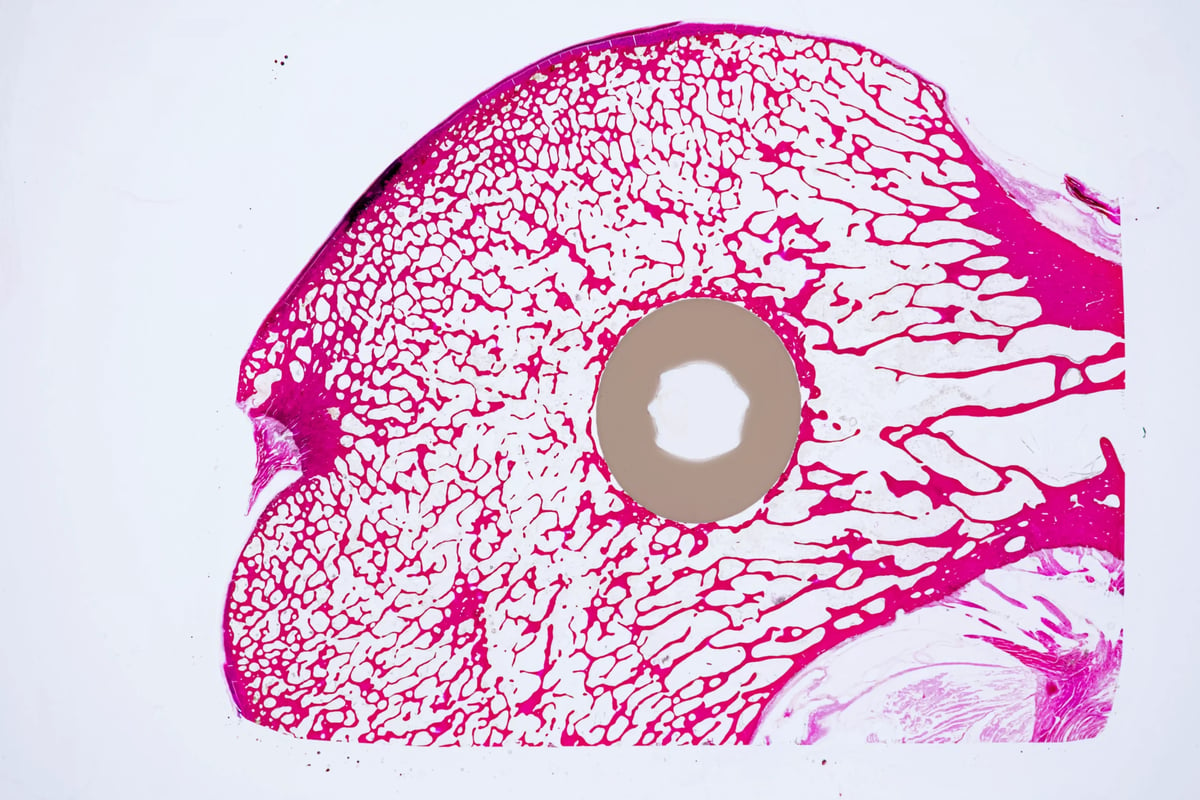
CARBON-FIBER REINFORCED PEEK FOR 3D PRINTED IMPLANTS
As the global needs in the medical industry continue towards more customization, digitalized and metal‑free solutions, the range of possibilities in high-tech medical plastics is boundless. This goes beyond human implants. In its future-oriented strategy for smart PEEK biomaterials, Evonik is also exploring uses for joint prostheses in veterinary as well as in human implants. Continued research and development in joint implants aim to reduce the need for revision surgery and long-term pain therapy.
Among other developments to come, Evonik is introducing a new carbon-fiber reinforced PEEK filament, for use in 3D printed medical implants. This smart biomaterial can be processed in common extrusion-based 3D printing technologies such as fused filament fabrication (FFF). The specialty chemicals company will present the new product for the first time at coming next medical technology and 3D printing related trade shows.
Dubbed VESTAKEEP® iC4612 3DF and VESTAKEEP® iC4620 3DF, the two available filaments feature 12% and 20% carbon fiber content, respectively. The two grades offer a choice of material depending on the required strength and flex properties of 3D printed implants such as bone plates and other reconstructive prostheses.
“By introducing the world’s first carbon-fiber reinforced PEEK filament for long-term medical implants, we continue to design biomaterials that open up new possibilities in today’s medical technology for patient-specific treatment,” says Marc Knebel, Head of Medical Systems at Evonik. “As passionate experts with decades of experience in polymer chemistry, we combine a unique set of competencies in materials science, manufacturing technologies and regulatory expertise to customers to accelerate time-to-market of new medical technologies for people’s lives beyond limits.”
In the future, as medical industry needs grow, PEEK-based biomaterials will continue to be a boon for medical plastics manufacturers operating in this exciting, high-growth field.
