MIT chemists apply living polymerization technique to 3D printing; objects whose chemical composition and mechanical properties can change.
What better way to optimize your 3D printing than by creating objects that can morph into new shapes? Or even take on new mechanical properties?
This is not an outlandish fantasy. A group of chemists from MIT have developed a technique that makes mutating 3D printed objects a reality. New polymers are added to a 3D printed object, altering the chemical composition and mechanical properties of the original gel material.
This technique is based off an approach called “living polymerization”. Ultimately, it allows you to 3D print an object and morph or grow the object’s material with light.
The MIT chemists first experimented with living polymerization for 3D printing several years ago; they set out to create adaptable 3D printed structures from materials that could grow, temporarily stop, and then reactivate later.
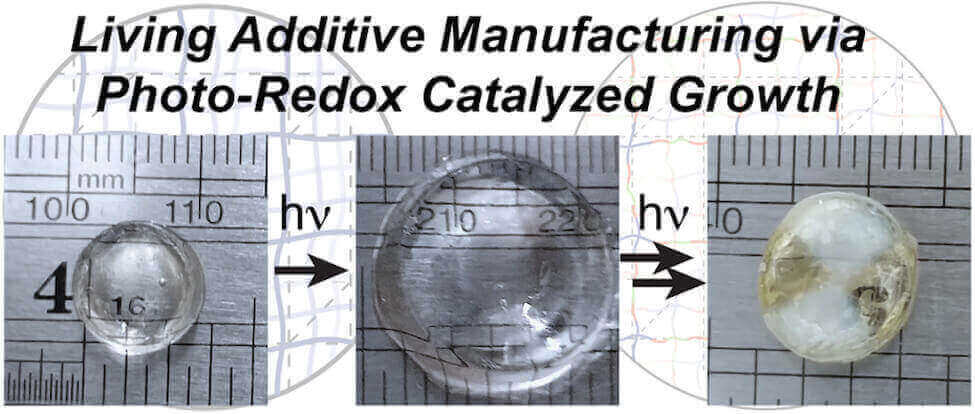
By 2013, the researchers had managed to stimulate growth of new features on 3D printed materials via ultraviolet light. This light source breaks apart the polymers to create free radicals, which binds to monomers and fuses with the material of the 3D printed object.
Living Polymerization Shows Incredible Potential
Jeremiah Johnson, the senior author of the study and MIT Firmenich Career Development Associate Professor of Chemistry, said:
“The advantage there is you can turn the light on and the chains grow, and you turn the light off and they stop. In principle, you can repeat that indefinitely and they can continue growing and growing.”
While the initial living polymerization technique was a step in the right direction, the reactive nature of the free radicals were difficult to command. It was too damaging to the material. So the research team developed a different type of polymer that reactivated with light in a more controlled manner.
These “thermo responsive gels” contain chemical groups called TTCs. The TTCs are stimulated by an organic catalyst that is powered by blue light from an LED. The reaction causes new monomers to attach to the TTCs, stretching them out and giving the material new properties.
The research, recently published in ACS Central Science, demonstrates that adding specific monomers can alter material properties like stiffness and water resistance. In addition, the research team fused two different objects together by directing the light source to the regions where the structures touched.
Overall, the MIT research shows incredible potential for adaptable 3D printing materials. By printing objects that can continuously be altered and infinitely grown, 3D printing technology just took one giant leap.

License: The text of "Living Polymerization: MIT Creates Adaptable 3D Printing" by All3DP is licensed under a Creative Commons Attribution 4.0 International License.